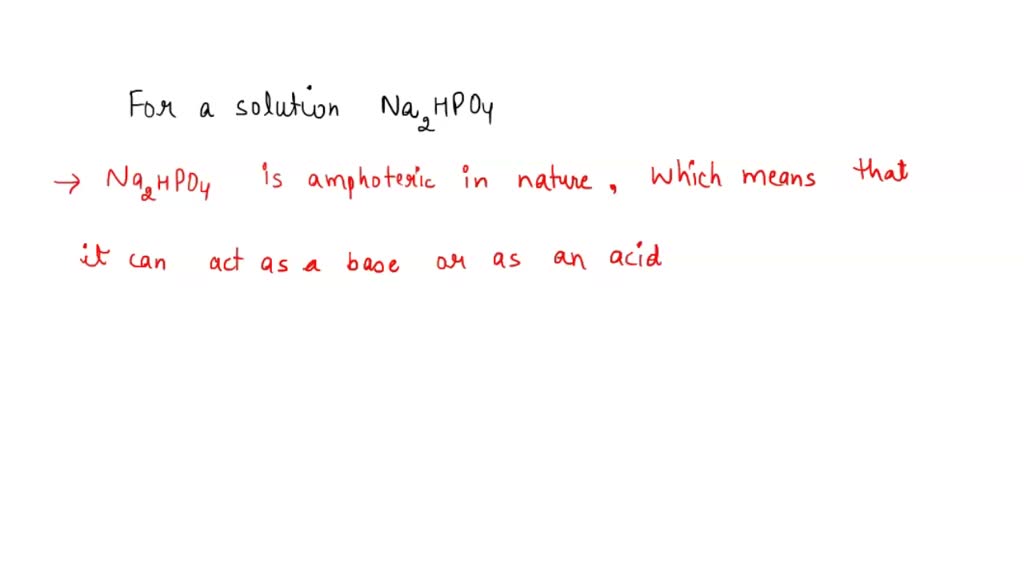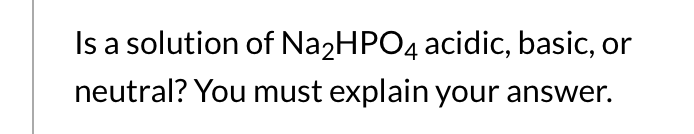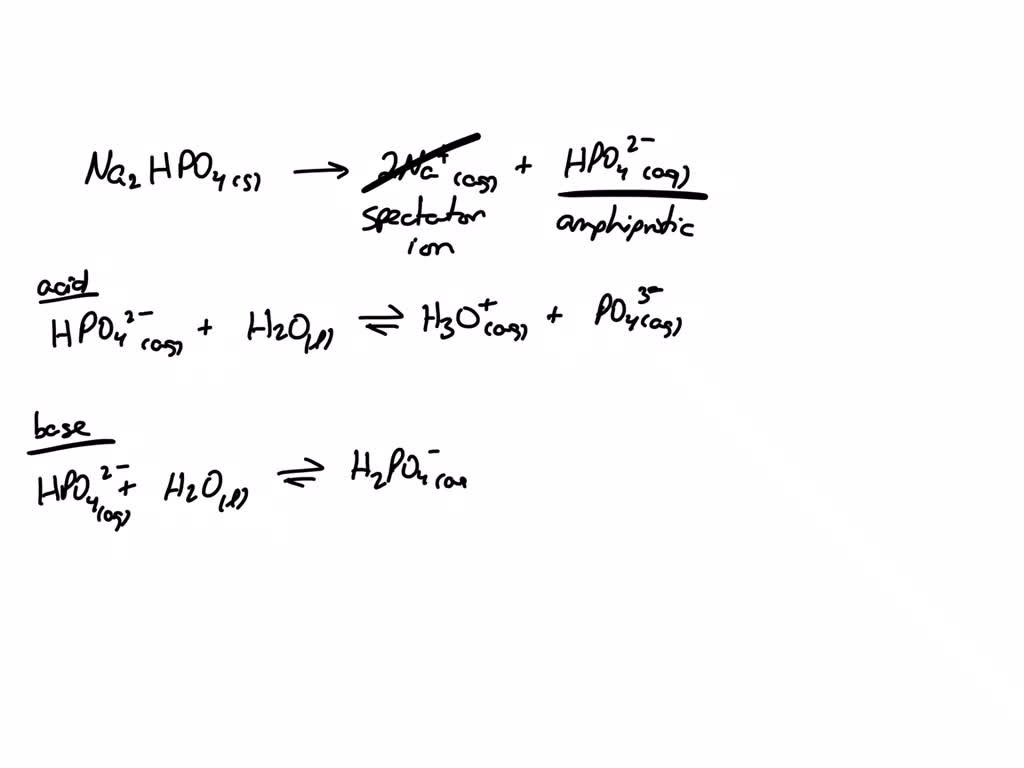
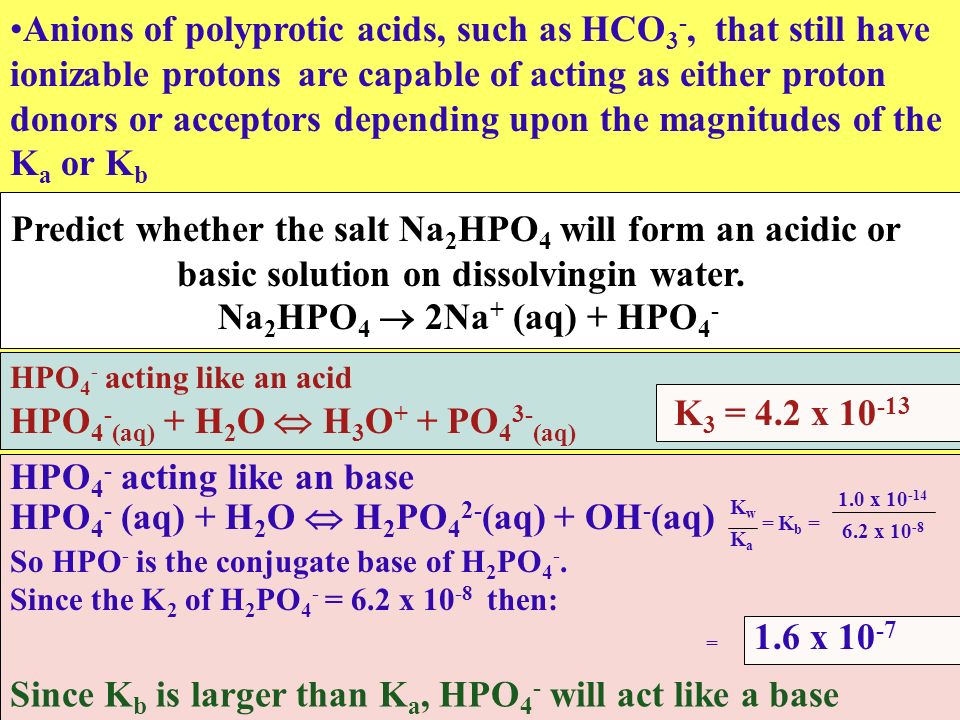
SOLVED: Predict whether the salt Na2HPO4 forms an acidic solution or a basic solution when dissolved in water.
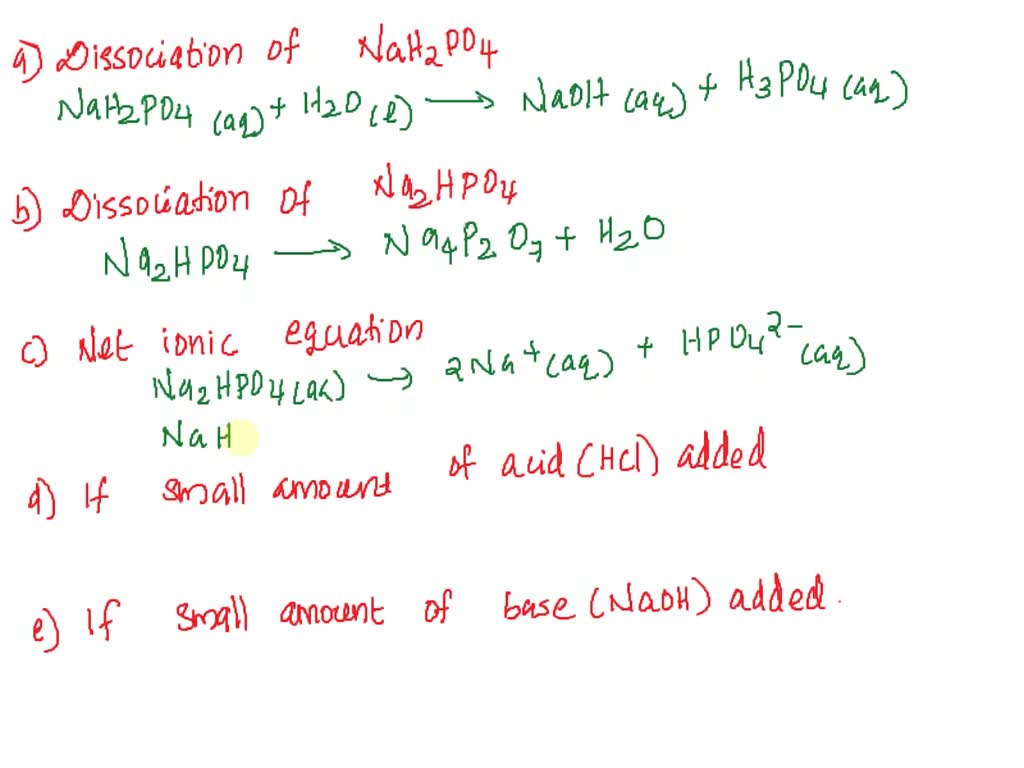
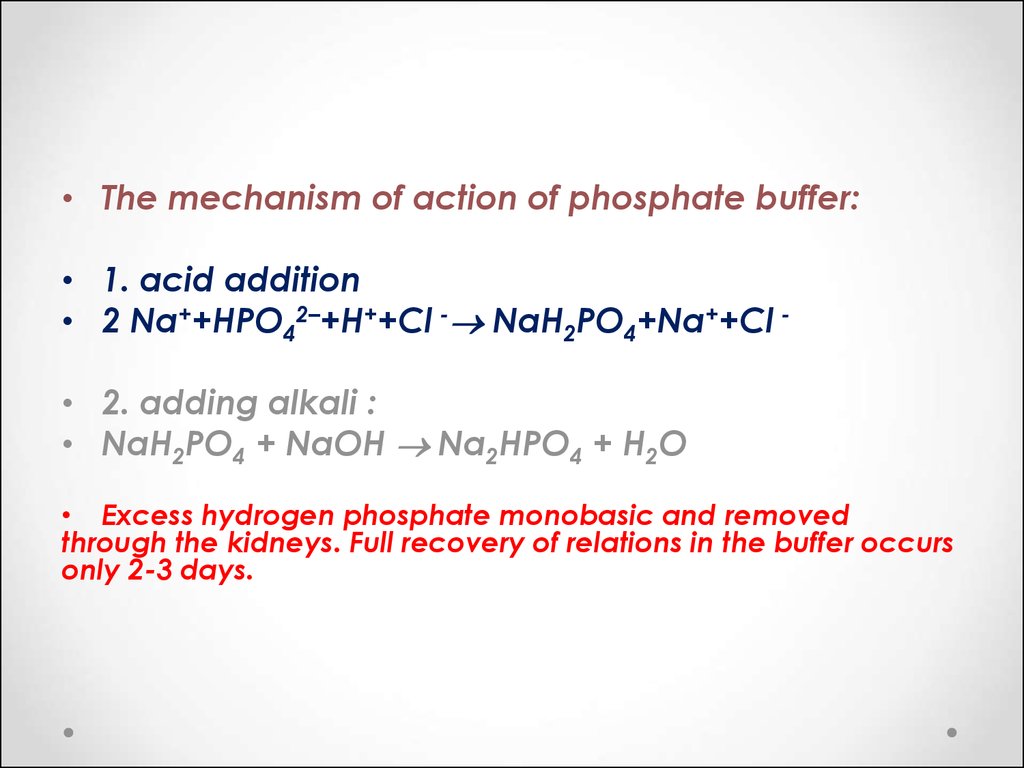
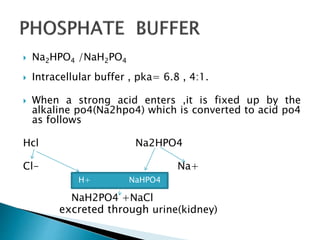
SOLVED: A buffer was made by mixing aqueous solutions of NaH2PO4 and Na2HPO4 together. This buffer is made by mixing two salts together. a. Write the balanced dissociation reaction for solid NaH2PO4

OneClass: Equal molar quantities of sodium hydroxide and sodium hydrogenphosphate (Na2HPO4) are mixed...
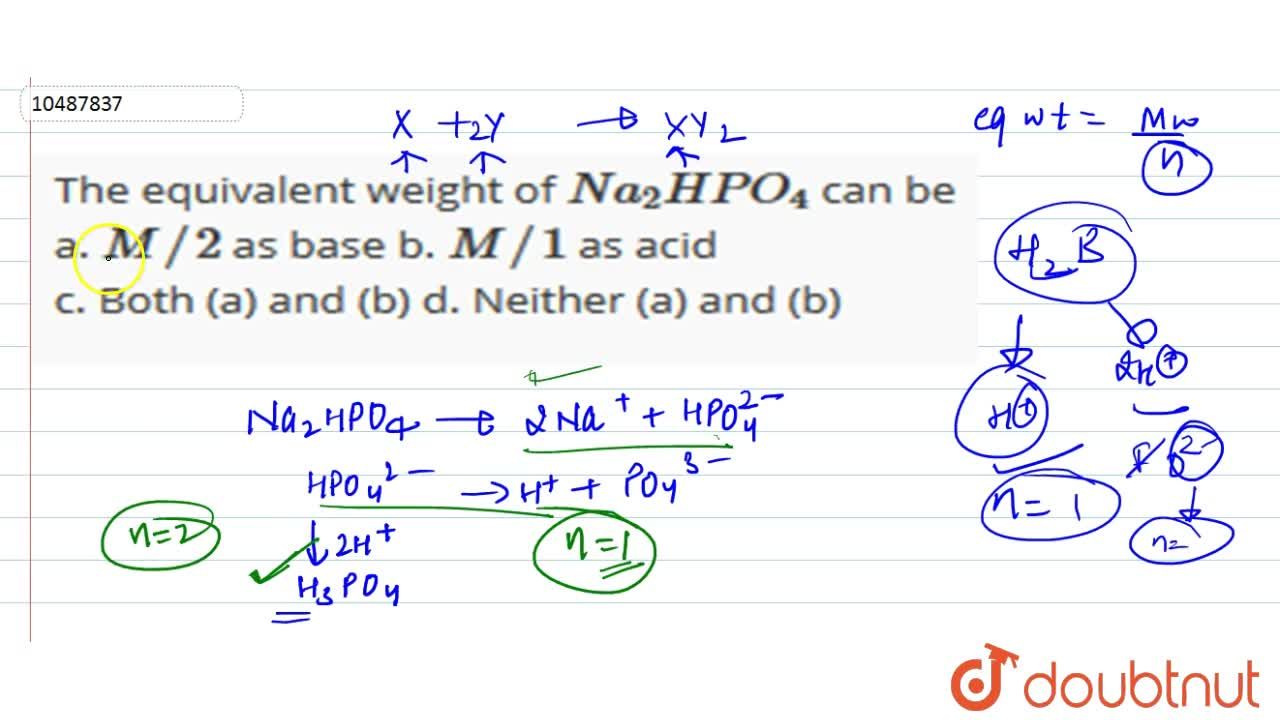
The equivalent weight of Na(2) HPO(4) can be a. M//2 as base b. M//1 as acid c. Both (a) and (b) d. Neither (a) and (b)

OneClass: 2. In the following reactions, label the acid, base, conjugate acid and conjugate base. (4 ...

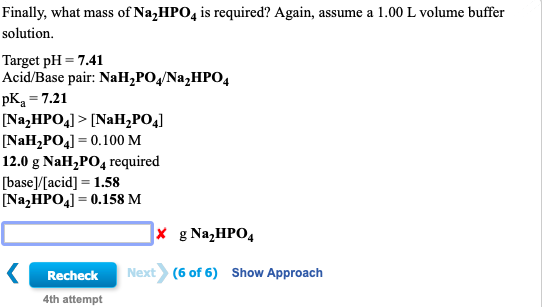
Calculate the pH of a buffer solution obtained by dissolving 25.0 g of KH2PO4(s) and 38.0 g of Na2HPO4(s) in water and then diluting to 1.00 L. | Homework.Study.com


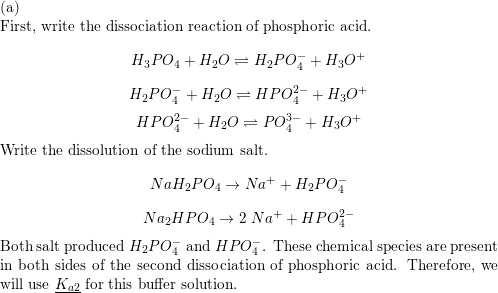


![An example of an acid salt is [CH(3)COONa//NaNO(3)//Na(2)HPO(4)//NaKCO(3)] An example of an acid salt is [CH(3)COONa//NaNO(3)//Na(2)HPO(4)//NaKCO(3)]](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/643651395_web.png)