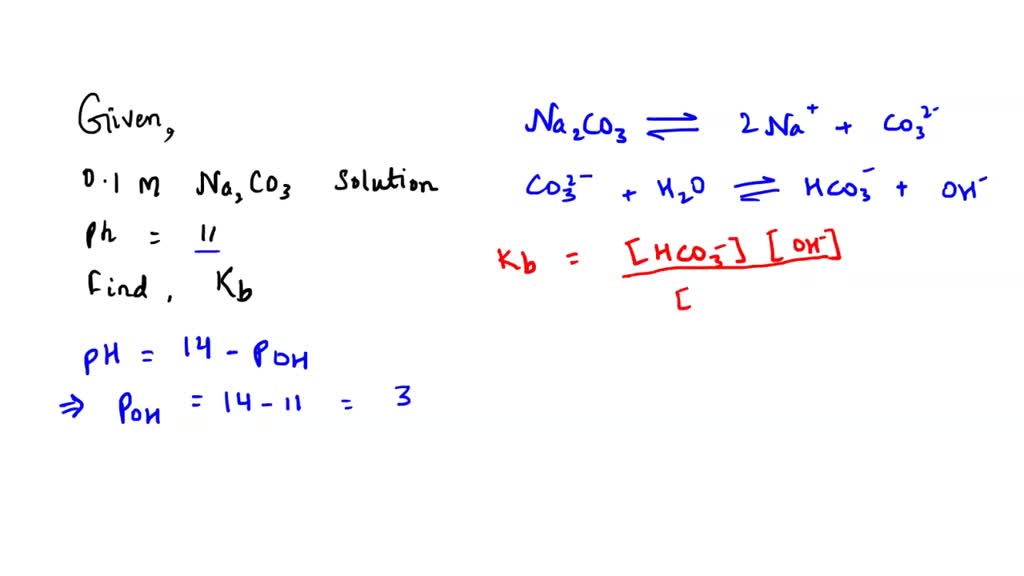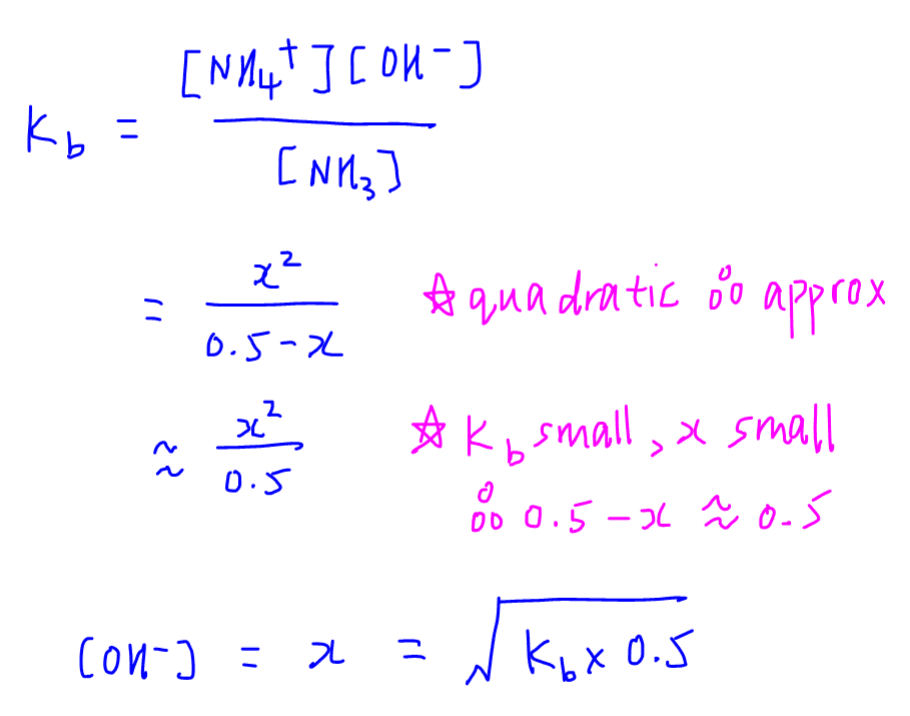Calculate the pH of the following mixture given Ka = 1.8 × 10^-5 and Kb = 1.8 × 10^-5 ( pKa = pKa = 4.7447 ) 50mL 0.05M NaOH + 50mL of 0.1M CH3COOH

pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems - YouTube

SOLVED: The larger Ka (smaller pKa), the smaller Kb (larger pKt): Stronger the acid, weaker its conjugate base, and vice versa Calculate Kb for the base F- anion if Ka = 6.8

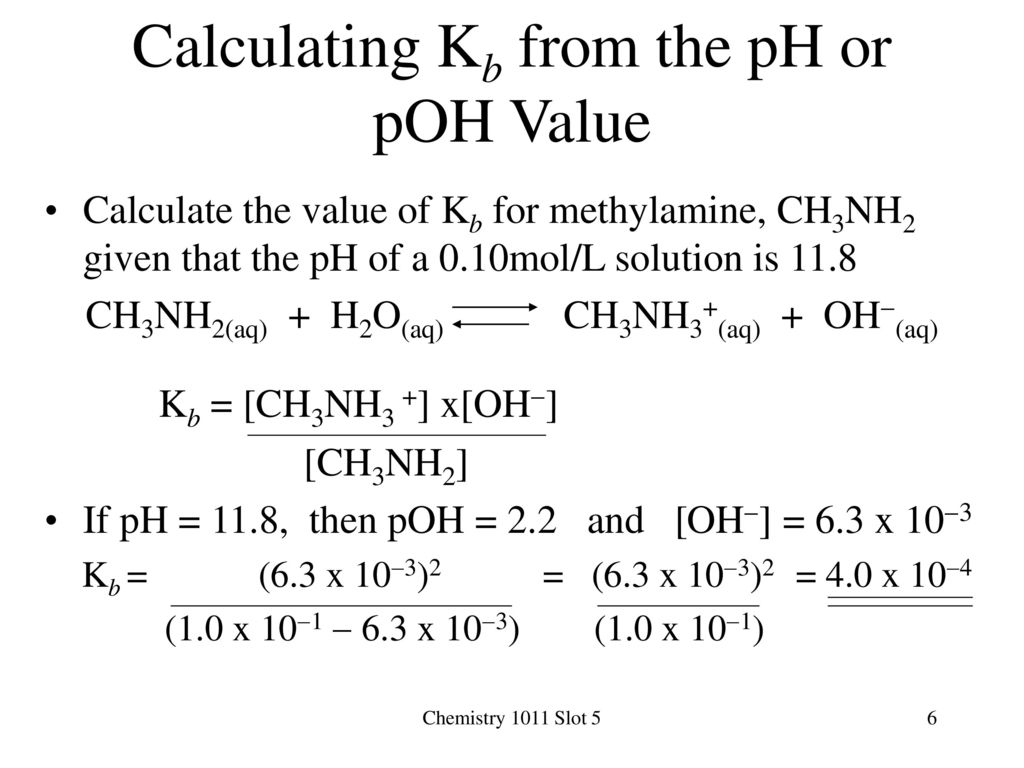











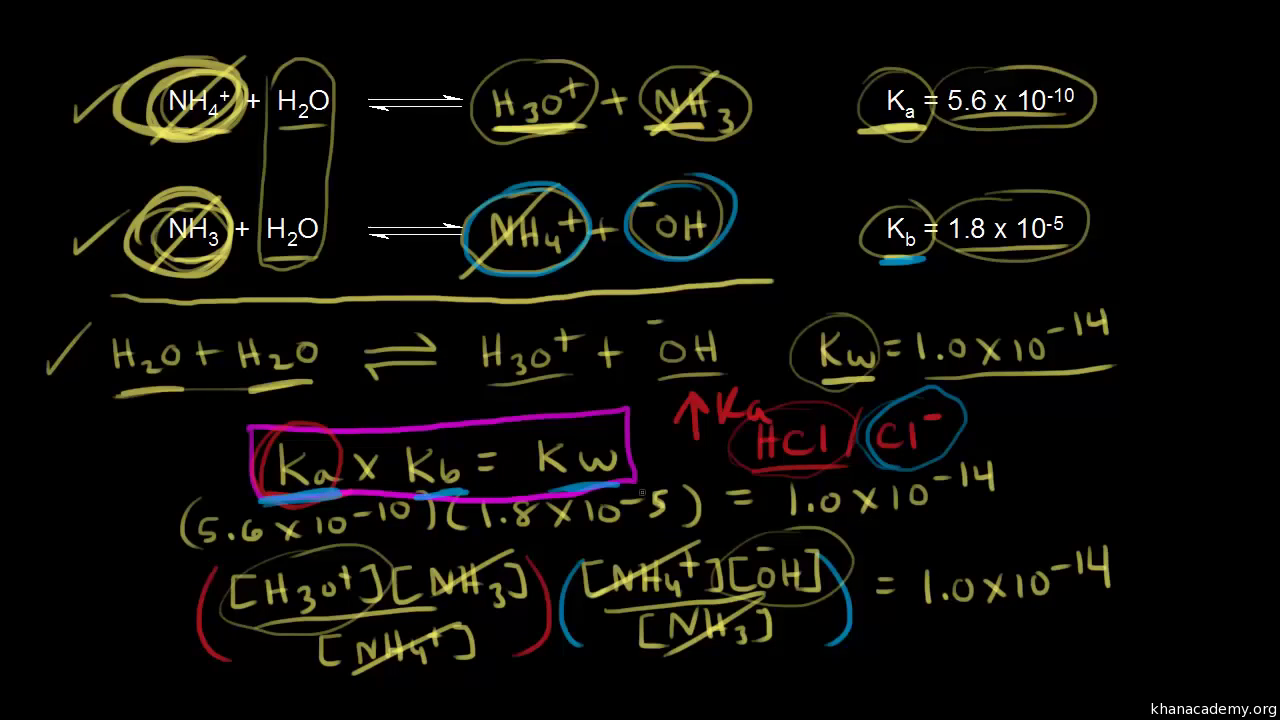




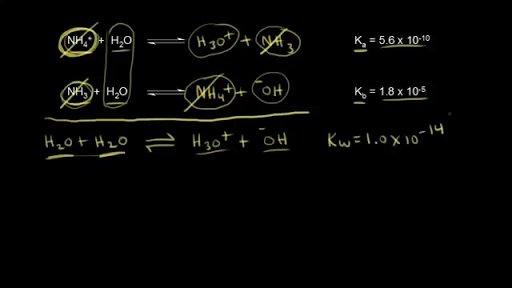
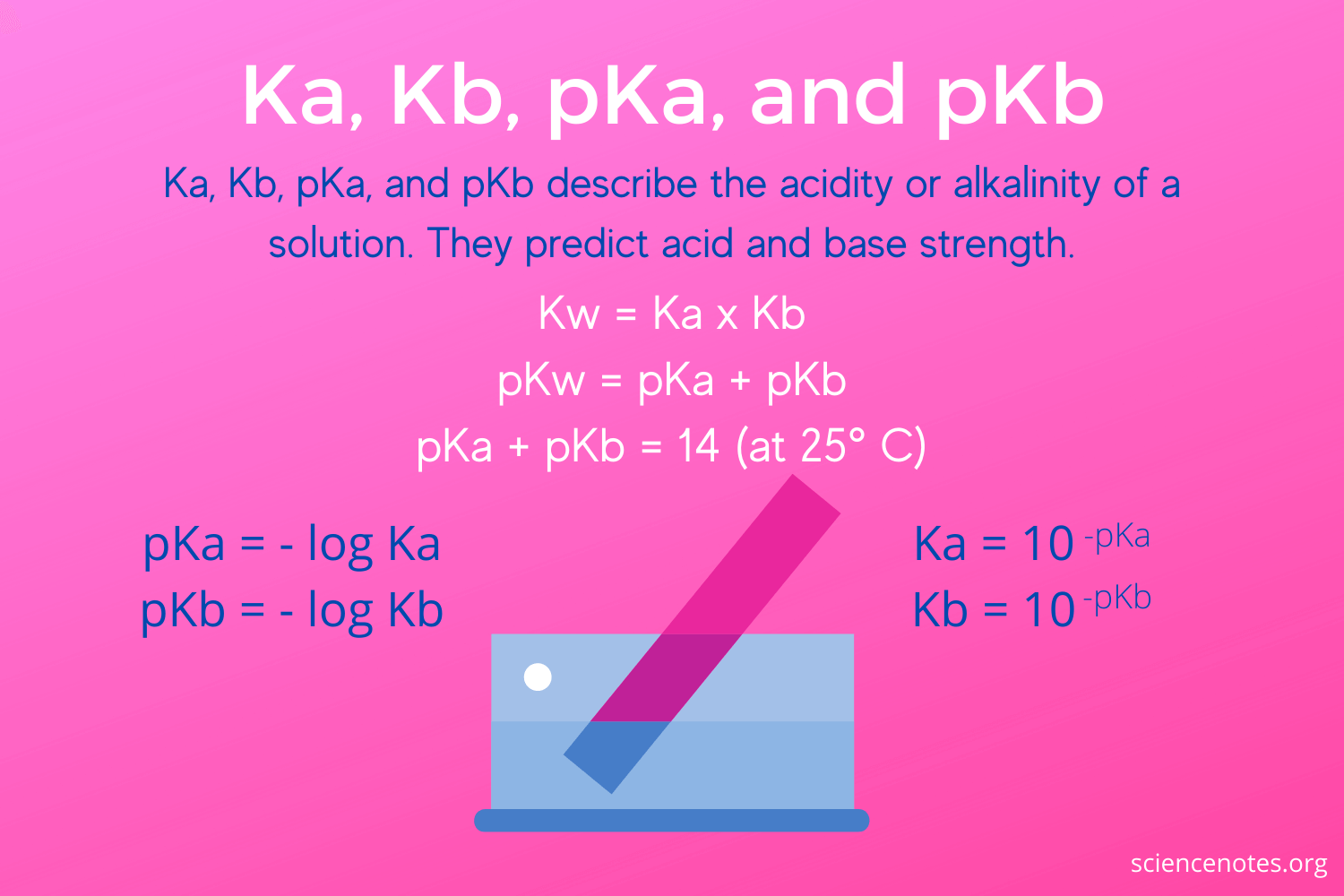
![Calculating [OH-], pH and pOH from Kb Calculating [OH-], pH and pOH from Kb](https://www.mi.mun.ca/users/pfisher/chemistry1011_135/img007.gif)