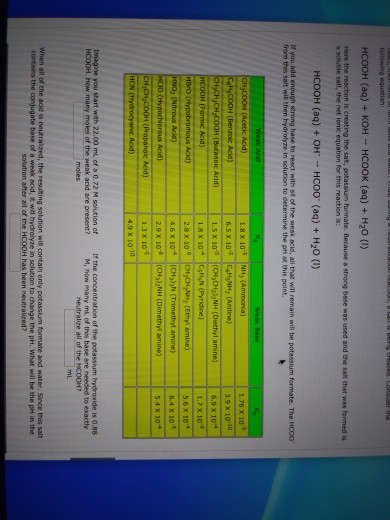
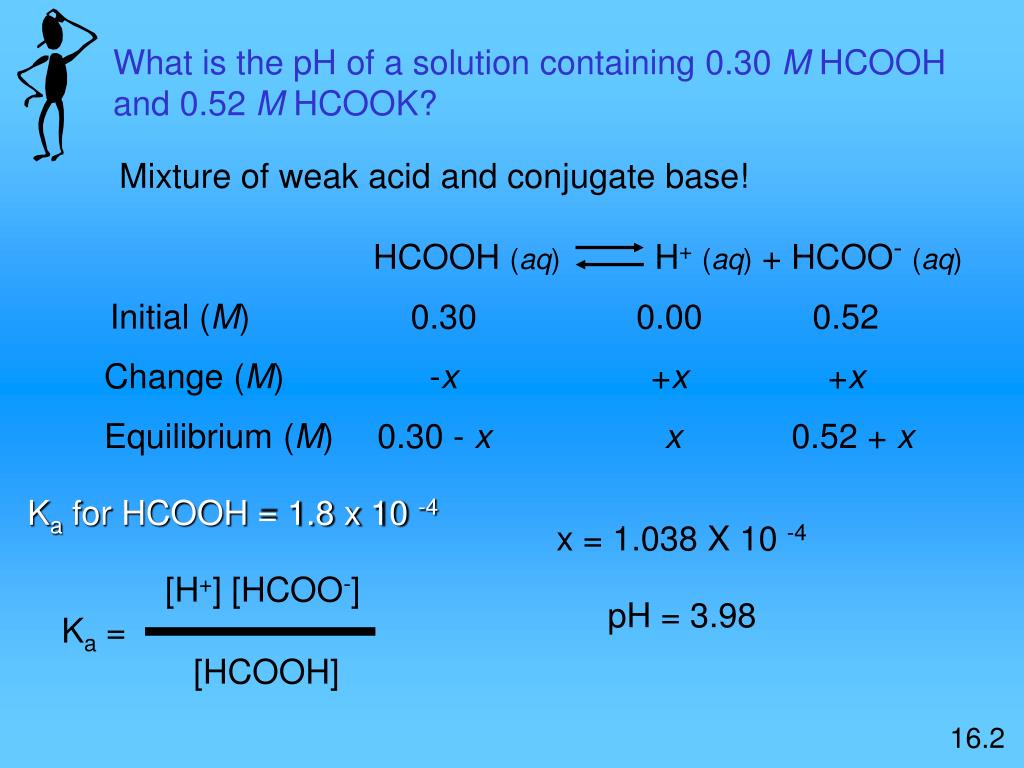
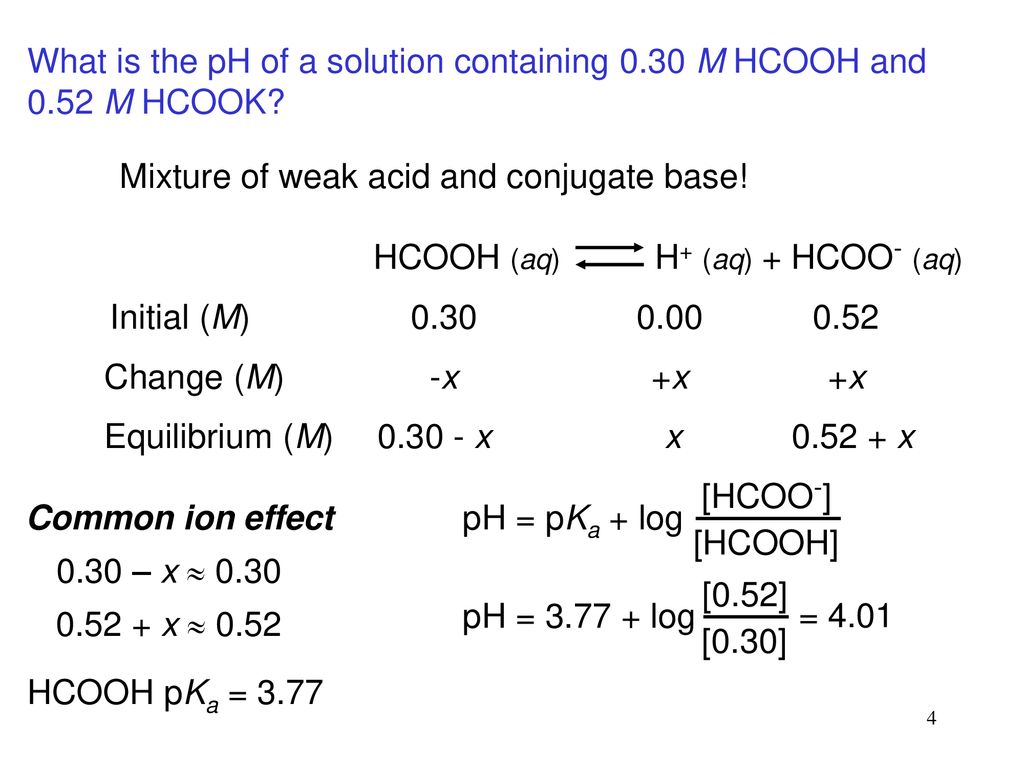
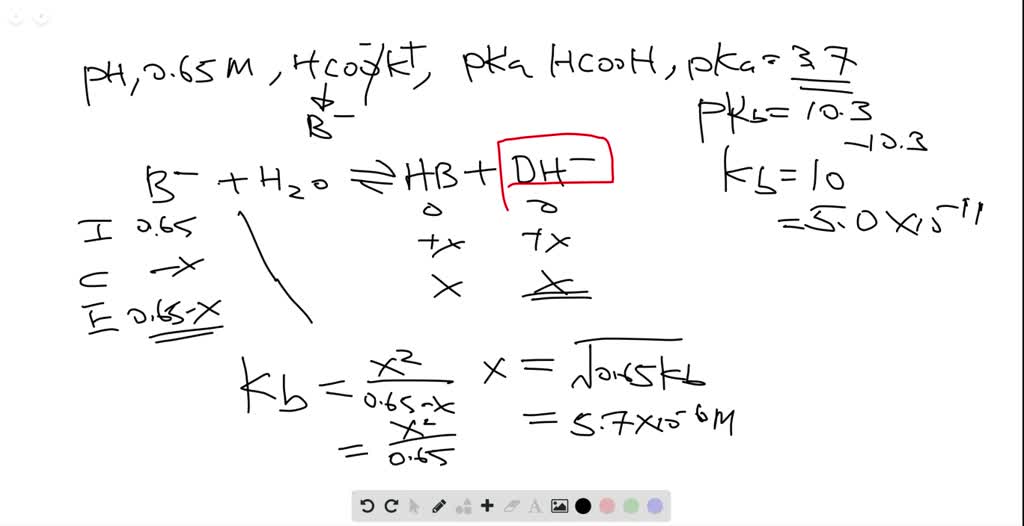
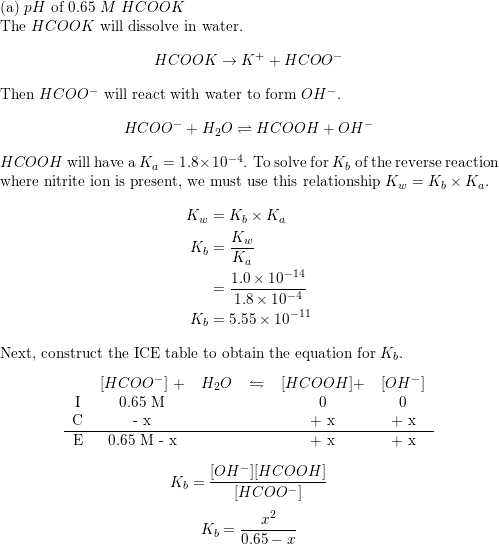In acid buffer solution (pH = 4.4), the ratio of concentrations of acid to salt is 2 : 1. The value of dissociation constant of weak acid may be:
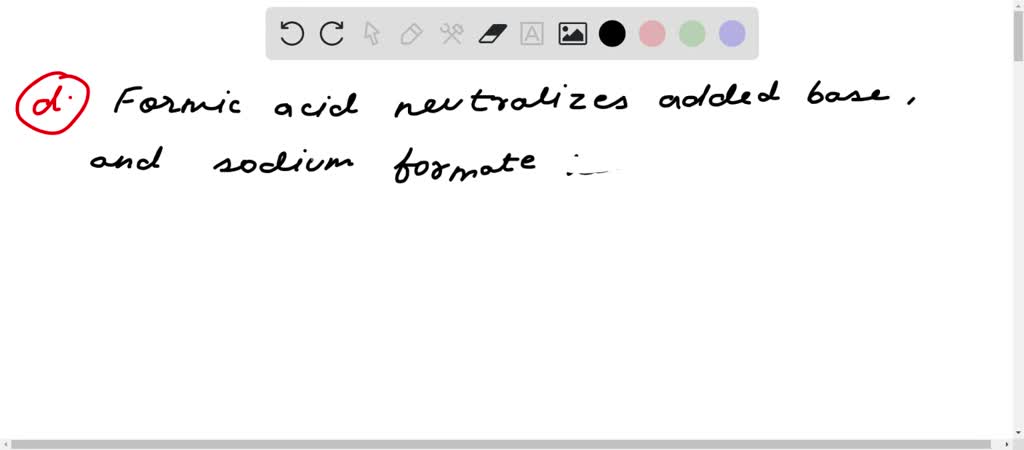
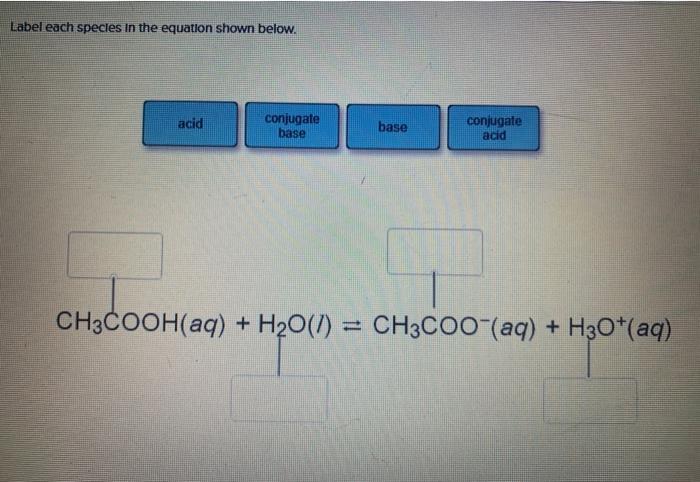
SOLVED: A buffer contains HCOOH (aq) and HCOOK (aq). Which statement correctly summarizes the action of this buffer? Both HCOOH (aq) and HCOOK (aq) neutralize added acid. Both HCOOH (aq) and HCOOK (

Acid-Base Equilibria Chapter HF( aq ) + H 2 O( l ) H 3 O + ( aq ) + F - ( aq ) Addition of NaF will shift the equilibrium to the _______because. - ppt download
Steady continuous dosage of FA. Reaction conditions: HCOOH (5 mmol),... | Download Scientific Diagram
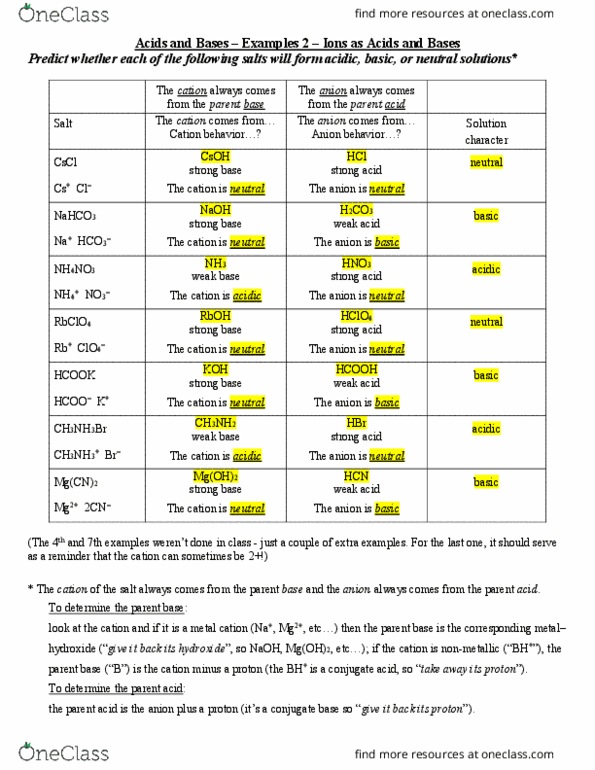
CHE 1302 Lecture Notes - Fall 2017, Lecture 12 - Equilibrium Constant, Hydrofluoric Acid, Rice Chart
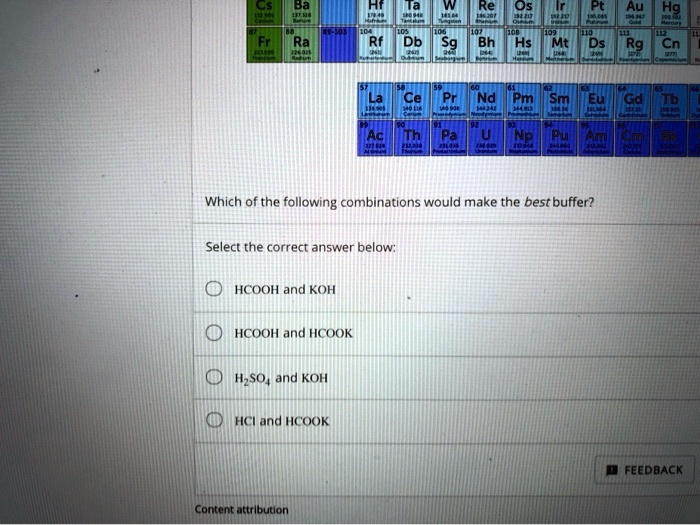
SOLVED: Os 6H Db Sg Hs Mt Ds Rg Cn Cel PN Which of the folllowing combinations would make the best buffer? Select the correct answer below HCOOH and KOH HCOOH and
In situ observation of [Mn-OOCH] by NMR. Reaction conditions: HCOOH (5... | Download Scientific Diagram
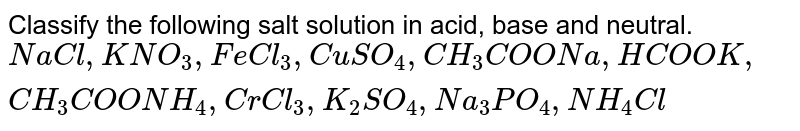
Classify the following salt solution in acid, base and neutral. NaCl, KNO3 , FeCl3 , CuSO4 , CH3COONa, HCOOK, CH3COONH4 , CrCl3 , K2SO4 , Na3PO4 , NH4Cl
![pH = - log [H + ] or pH = - log [H 3 O + ] Example: If [H + ] = 1 X pH = - log 1 X pH = - (- 10) pH = 10 What would be the pH of a. - ppt download pH = - log [H + ] or pH = - log [H 3 O + ] Example: If [H + ] = 1 X pH = - log 1 X pH = - (- 10) pH = 10 What would be the pH of a. - ppt download](https://images.slideplayer.com/26/8472866/slides/slide_40.jpg)
pH = - log [H + ] or pH = - log [H 3 O + ] Example: If [H + ] = 1 X pH = - log 1 X pH = - (- 10) pH = 10 What would be the pH of a. - ppt download
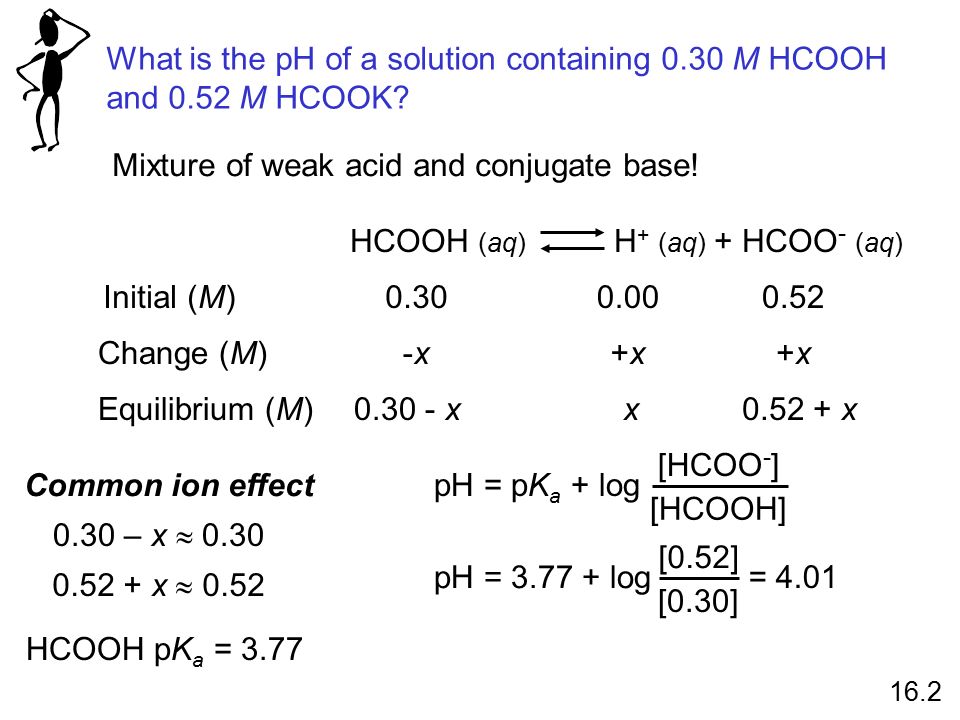
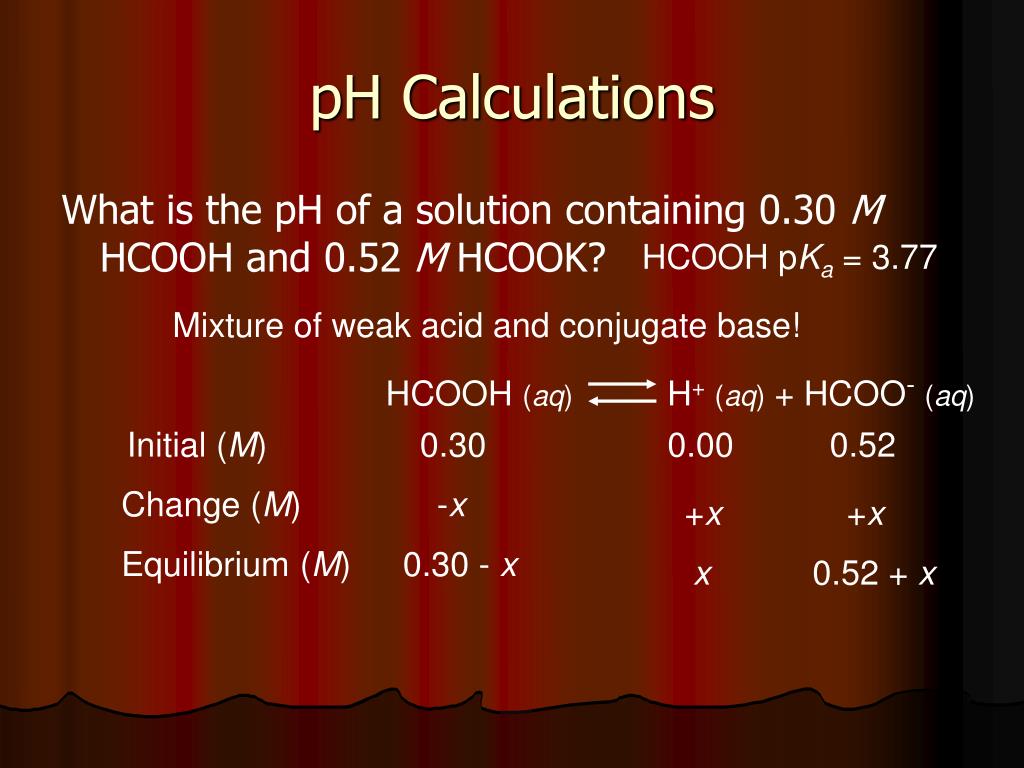
A 0.05M HCOOH and 0.1M HCOOK buffer solution was diluted 100 times. How did pH change and why? - Quora

Interconversion between CO2 and HCOOH under Basic Conditions Catalyzed by PdAu Nanoparticles Supported by Amine-Functionalized Reduced Graphene Oxide as a Dual Catalyst | ACS Catalysis