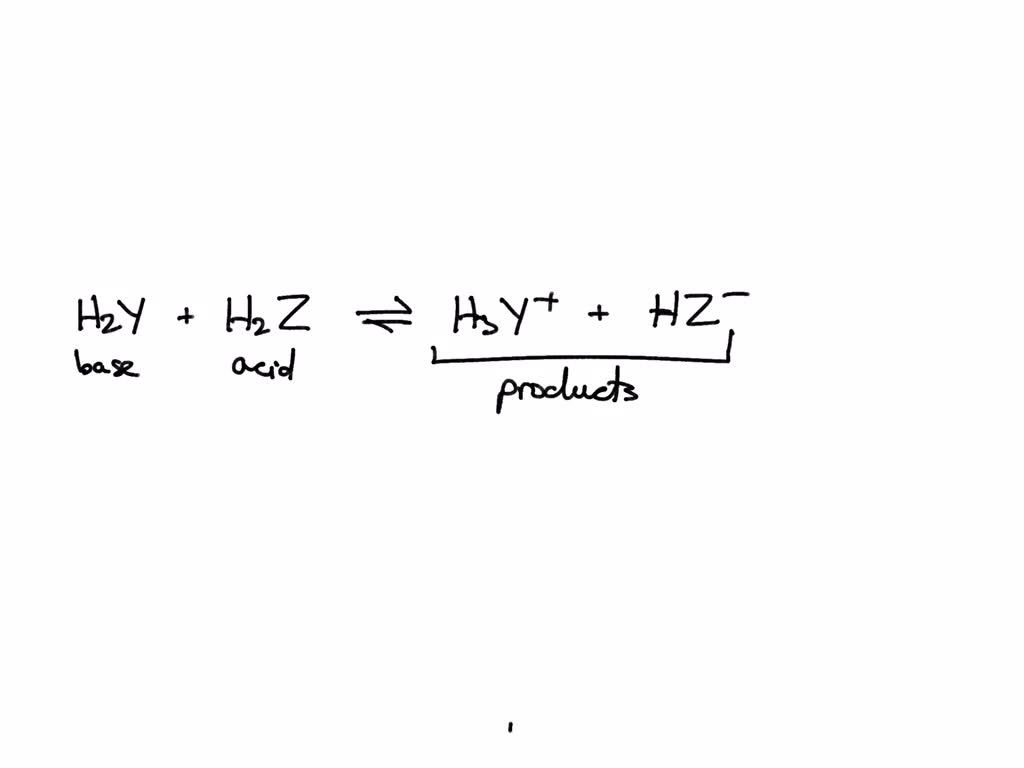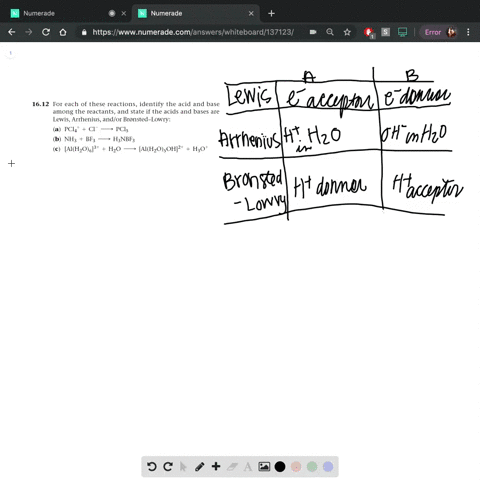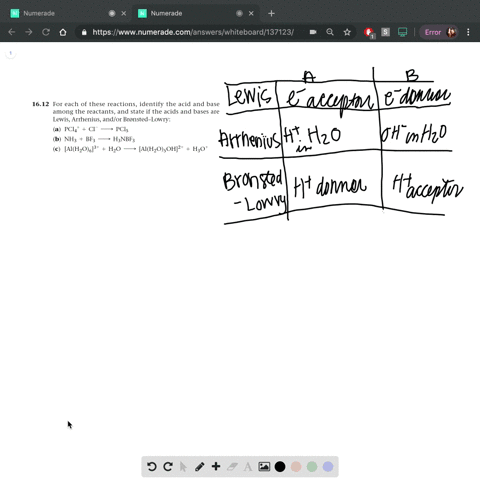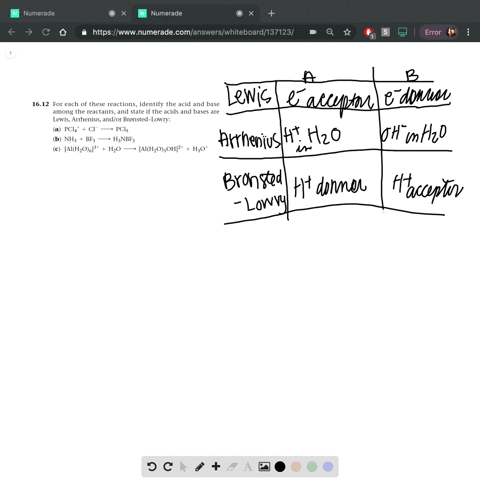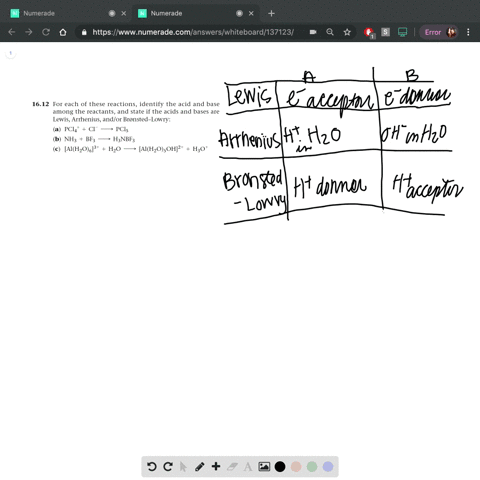
SOLVED: In the reaction between H2Y - and H2Z - , if H2Y - acts like a base, and H2Z - acts like an acid, the products of the reaction are: A)

acid base - How to predict the color of a pH indicator using Le Chatelier's principle - Chemistry Stack Exchange

Highly Selective Surface Lewis Acid−Base Reaction: Trimethylamine on Si(100)c(4×2) | The Journal of Physical Chemistry B

MEP maps of isolated TrHX, TrH2X and H2Y molecules (Tr = Ga, In; X = F,... | Download Scientific Diagram

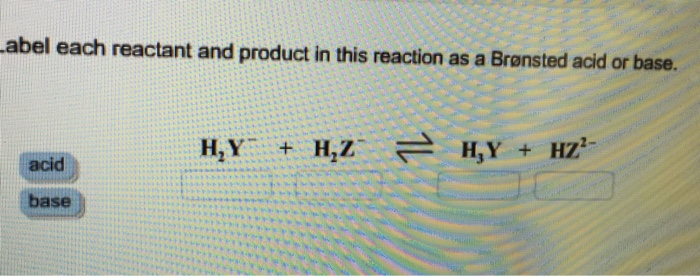
SOLVED: Identify each reactant and product in this reaction as a Bronsted acid or base. Hz Y- + HzZ acid base H; Y + HZ2 - base acid Answer Bank base acid

Complete a net ionic equation for each proton-transfer reaction using curved arrows to show the flow of electron pairs. Write Lewis structures for all starting materials and products, label the original acid
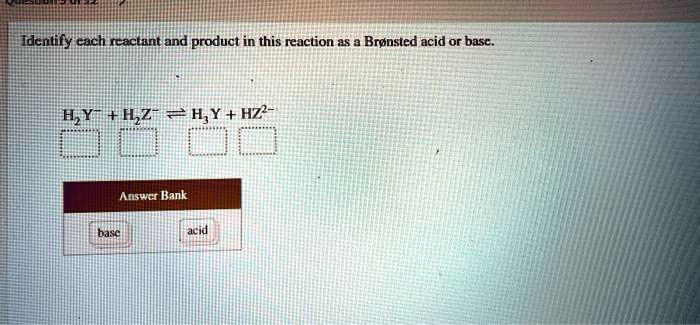
SOLVED: Identify each reactant and product in this reaction as a Brónsted acid or base? H2Y- + H2Z- <—> H3Y + HZ2- Identily cach ractant and product in this reaction as Bronsted
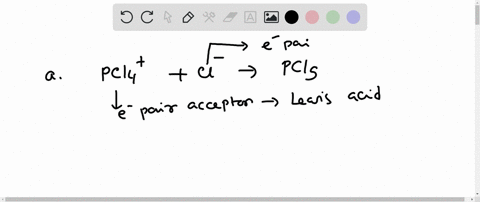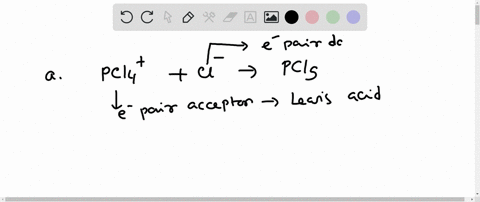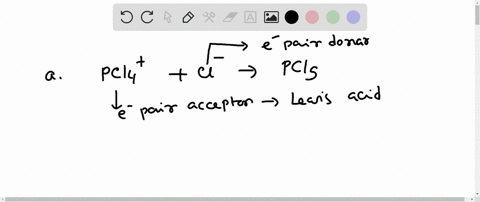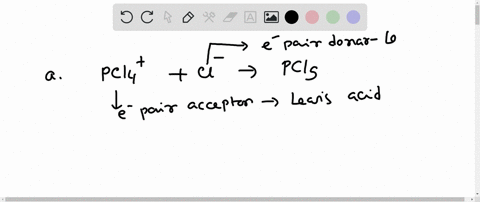
SOLVED: In the reaction between H2Y - and H2Z - , if H2Y - acts like a base, and H2Z - acts like an acid, the products of the reaction are: A)
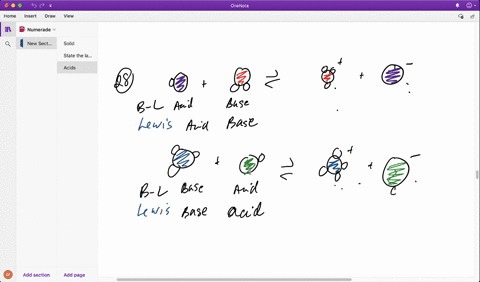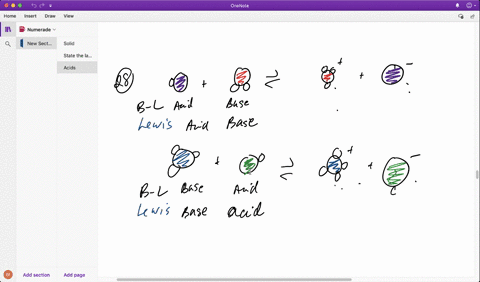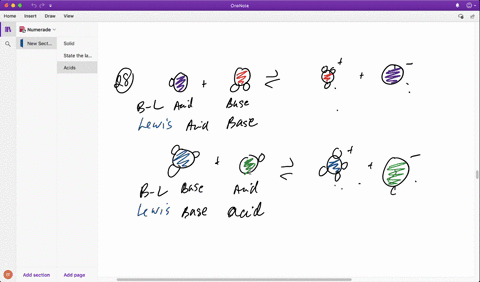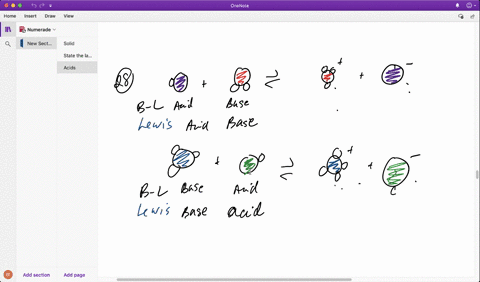
SOLVED: In the reaction between H2Y - and H2Z - , if H2Y - acts like a base, and H2Z - acts like an acid, the products of the reaction are: A)
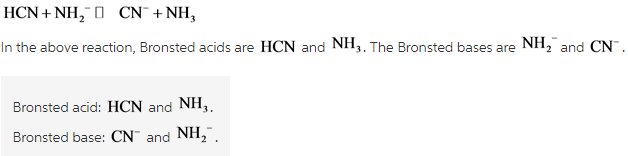
Label each reactant and product in this reaction as a Bronsted acid or base - Home Work Help - Learn CBSE Forum

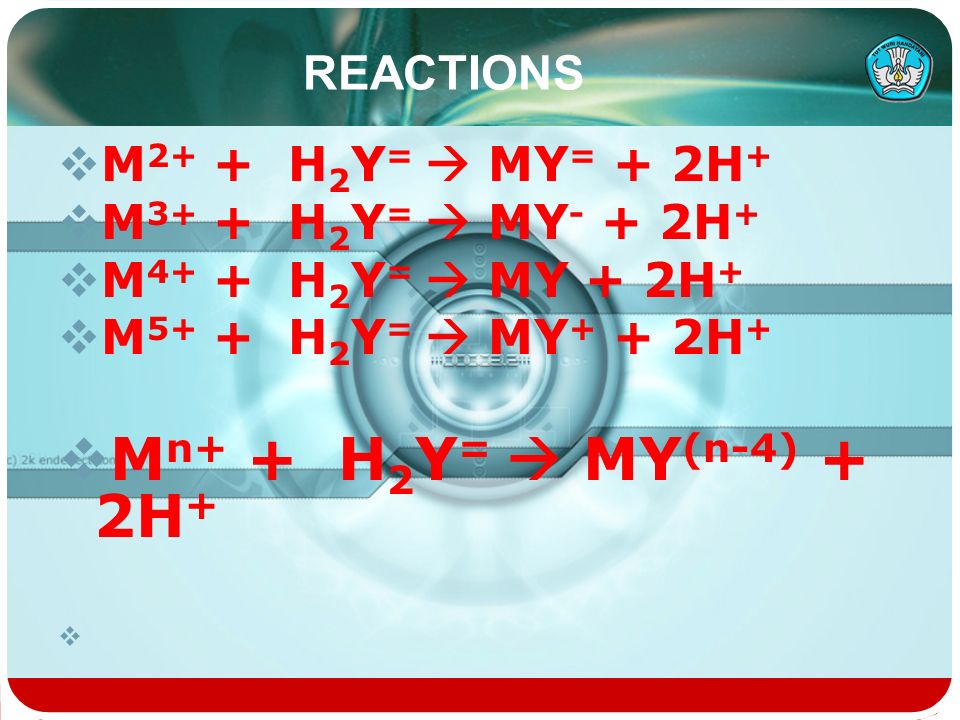
25.0cm<sup>3</sup> of 0.12M potassium hydroxide solution required 30.0cm<sup>3</sup> of a solution of a dibasic acid (H<sub>2</sub>Y) for complete neutralization. The acid contained 3.15g per 500cm<sup>3</sup>...

Nonaqueous Chemistry of Group 4 Oxo Clusters and Colloidal Metal Oxide Nanocrystals | Chemical Reviews

SOLVED: 30.At what pH would you expect the aminoacid prolinePto be fully protonated?pK=1.952,pK2=10.640 A.1.952 B.1.750 C.2.500 D.10.640 E.7.000 31.Given pKvalues of citric acid2-hydroxypropane-1,2,3-tricarboxylic acid,pK=3.128,pK=4.761,and pK3= 6.396 ...